The passivation of stainless steel bearings is a pivotal process that impacts the efficiency, safety, and sustainability of various industrial operations, making it an essential practice in modern manufacturing and engineering. In this article, we'll delve deeper into the intricacies of passivation, its steps, and its significance in the performance of stainless steel bearings.
For more about Characteristics, Uses and Advantages of Stainless Steel Bearings, please click here!
Stainless steel bearings inherently possess anti-corrosion properties, primarily attributed to their high content of chromium, nickel, and other alloying elements. These elements oxidize to form a passivation film, a natural barrier against rust.
But it's essential to understand that while stainless steel bearings resist rust, they aren't entirely rust-proof. The innate passivation layer on stainless steel bearings is only between 1 to 3 nanometers thick, offering a certain level of protection.
Still, challenges like welding, scratching, and assembly can damage this oxide film. When damaged, harmful iron particles or ions may surface, accelerating rusting, especially in demanding applications like coastal areas, food processing, or chemical plants.
Passivation is a non-electrolytic process applied to stainless steel bearings post-manufacture to enhance their resistance to corrosion and rust.
Stainless steel's composition typically includes iron, chromium, and various other non-ferrous metals, varying with the alloy. Since rust formation necessitates iron, eliminating iron molecules from the surface results in a dominance of chromium molecules, inherently inert by nature. This concentration of chromium leads to the formation of a robust, non-reactive, and passive layer, significantly impeding rust development.
Typically, following the passivation of stainless steel bearings, a dense passivation film with a thickness ranging from 1 to 10 nanometers forms on the surface.
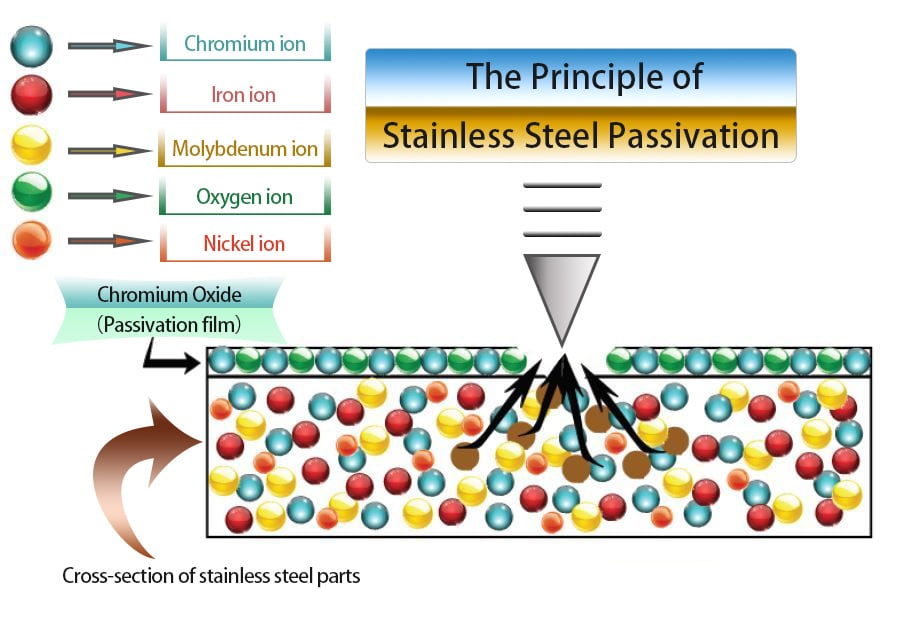
Fig 1 The Principle of Stainless Steel Passivation
The passivation process is a critical post-manufacturing treatment for stainless steel bearings, designed to enhance their corrosion resistance and longevity. This process involves several key steps:
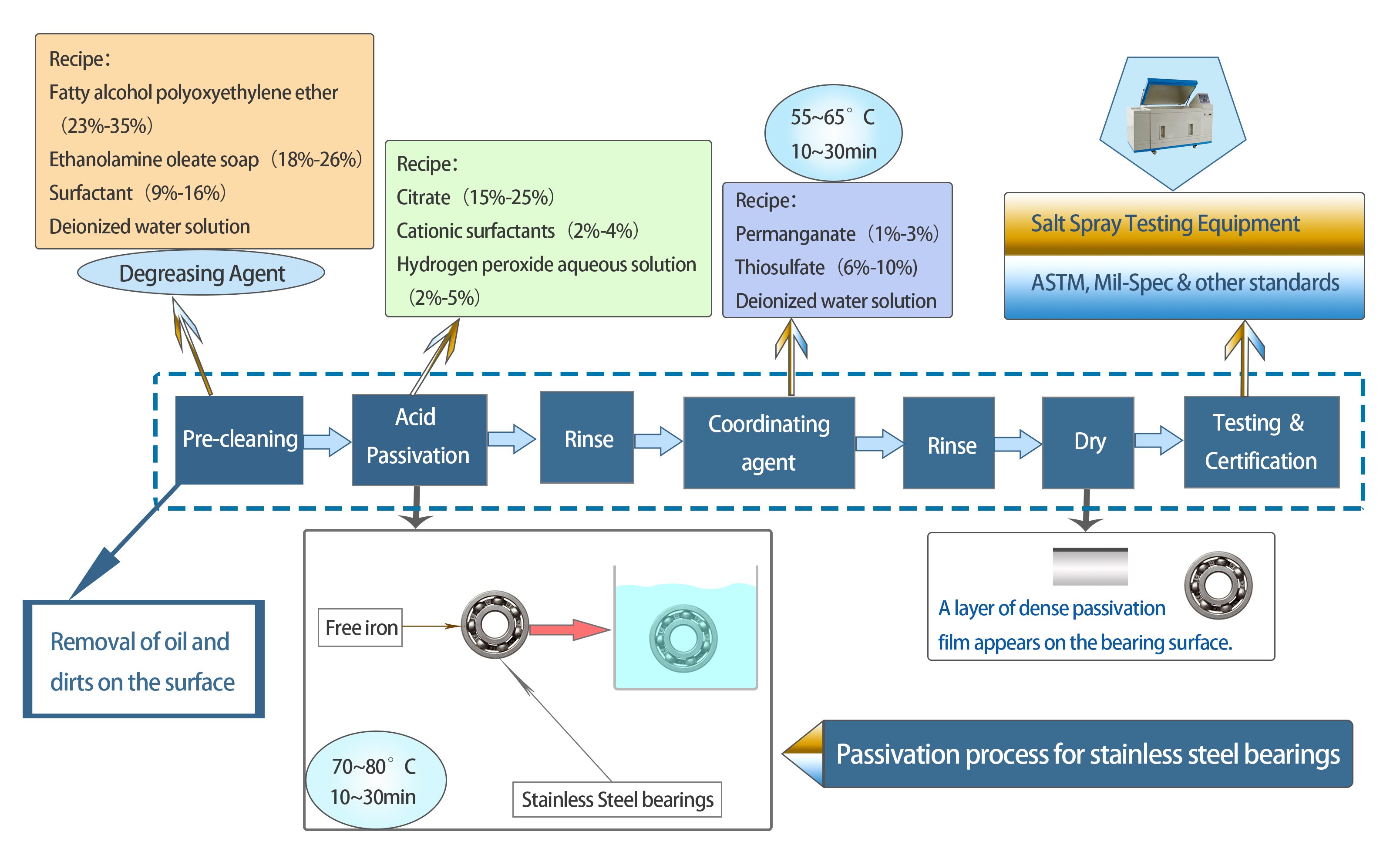
Fig 2 Passivation Process of Stainless Steel Bearings
Stainless steel bearings are immersed in a cleaning solution, often an alkaline solution or solvent-based cleaner, to break down and wash away residues. Ultrasonic cleaning can also be employed to dislodge stubborn particulates.
To avoid any chemical reactions with the passivation solution, bearings are rinsed using deionized or distilled water. This step might be repeated multiple times to ensure a thorough rinse.
Stainless steel bearings are immersed in an acid solution. Citric acid or nitric acid are the most commonly used agents for passivation.
The duration of immersion, acid concentration, and temperature are precisely controlled based on the stainless steel grade and intended application.
This step aims to remove free iron from the stainless steel bearing surface, which can be potential sites for corrosion. The removal of free iron enhances the natural corrosion resistance of stainless steel.
Use deionized or distilled water to remove residual acid before agent application.
After rinsing again, stainless steel bearings are treated with the coordinating agent which aids in forming a protective layer on the bearing's surface, further ensuring its protection against potential corrosion.
Use deionized or distilled water to wash off excess agent.
After using nitric acid for passivation, the acid needs to be neutralized to halt its activity and ensure safety. Stainless steel bearings are immersed in a neutralizing bath, typically a sodium bicarbonate (baking soda) solution.
To ensure that any residual acid or neutralizing agents are thoroughly washed off. Stainless steel bearings are again rinsed with deionized or distilled water, ensuring all chemical traces are removed.
To prevent water spots, which can be potential sites for corrosion, stainless steel bearings are dried using warm air blowers or placed in an oven with controlled temperature settings.
Purpose: Verify the effectiveness of the passivation process and ensure compliance with industry standards.
Method: Several tests can be employed:
Water immersion test: To check for signs of rusting or staining.
Salt spray test: Bearings are exposed to a salty mist to evaluate their resistance to corrosion.
Copper sulfate test: Mainly used for 300 series stainless steels to detect the presence of free iron.
Post successful testing, bearings are certified as per industry standards like ASTM A967, AMS 2700, or others as applicable.
To ensure the bearings remain contamination-free post-passivation, stainless steel bearings are packaged in corrosion-resistant packaging materials, often with desiccants, to keep them dry and ready for use or shipment.
While the above steps give a detailed overview of the passivation process, it's crucial to note that the specifics can vary based on the stainless steel grade, its intended use, and the industry standards to which it must comply.
This case study focuses on the remarkable impact of passivation on the corrosion resistance of stainless steel bearings.
HIY's 440 stainless steel bearings, a subject of this study, offer a clear example. Initially, these bearings could endure only 24 hours in a salt spray test without any treatment. However, post-passivation, their resistance to such corrosive conditions improved significantly, with the bearings now withstanding up to 168 hours in the same test.

Fig 3 Comparison of unpassivated and passivated stainless steel bearings after 168-hour salt spray test
This stark contrast demonstrates how passivation can substantially increase the lifespan and reliability of stainless steel bearings in corrosive environments.
In fact, about the salt spray test time, there is not only one standard. Different clients have different requirements, such as salt spray with 48 hours, 72 hours, 96 hours, 120 hours, 168 hours and so on. The specific salt spray test time can be located according to the actual working conditions.
Conclusion
For industries relying on the longevity and reliability of stainless steel bearings, the passivation process offers an added layer of protection against the harsh elements.
2024-09-06